Remdesivir Treatment Update (Lecture 64)
本文由‘中国推动’学者、江南大学药学院石漱新同学编辑整理。
Welcome to another MedCram COVID-19 update.
This update is for April 30th 2020 and we’re also looking forward to our first live webinar today 4 p.m. eastern time, 1 p.m. Pacific time. If you’re in the UK, that will be 9 p.m. In India, that’ll be 1:30 in the morning. And in Sydney Australia that will be at 6 o’clock in the morning. And during that webinar, we’re going to be talking about the path
of physiology, the epidemiology, diagnosis and treatment of COVID-19. We will see you there for that.
This is going to be a little bit more of an abbreviated update and we’re going to talk about the news of the day, which of course is Remdesivir. So this article was published on April 29th, 2020 in the Lancet and it’s a study out of China at ten hospitals. Let’s talk about the findings in this Remdesivir study.
They actually wanted to include many more patients than they were able to. And part of the reason for that was the pandemic in China was brought under control so quickly that they were unable to recruit the number of patients in their studies. So that’s the first thing that you should know about this study. The second one is that anybody with symptoms up to 12 days old, we’re able to be enrolled in the study. The two endpoints in the study was number one, time to
recovery and number two, mortality. And we have the Remdesivir and the placebo group. Now there was 237 subjects in the study and the time to recovery in the Remdesivir group was 21 days. And in the placebo group, it was 23 days. And this was not statistically significant. The mortality in the Remdesivir group was 14%. And in the placebo,
it was 13%. Again, not statistically significant.
And so this led the authors of the study to say “no statistically significant benefits were observed for Remdesivir treatment beyond those of standard of care treatment. Our trial did not attain the predetermined sample size because the outbreak of COVID-19 was brought under control in China. Further studies of Remdesivir, including earlier treatment in patients with COVID-19 and higher-dose regimens or in combination with other antivirals or SARS-Cov-2 neutral
ising antibodies in those with severe COVID-19 are needed to better understand its potential effectiveness.” So this was a disappointing result.
Now interesting, on the very same day, April 29th, the NIH released this news article “NIH clinical trial shows Remdesivir accelerates recovery from advanced COVID-19”. This news article is referencing a United States study that was looking at the same medication. So let’s talk about the results of this study.
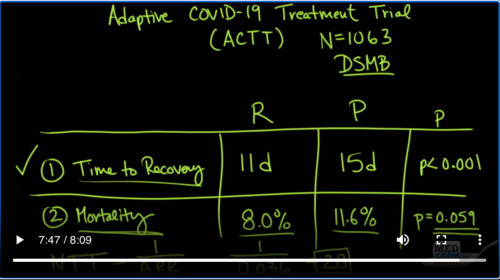
This was the adaptive COVID-19 treatment trial in the United States, otherwise known as ACTT. And they did their power analysis to try to figure out how many subjects they would need in their study to show statistical significance. And the number that they came up with was 1063. But as with all trials of this size, they do something called the data safety monitoring board, looks
at the study before it’s completed, sort of halfway through or even before that. And they do an interim data analysis. And the reason why they’re doing that is because there may be a possibility that this medication may have a stronger effect than the power that they have estimated and if that’s the case, if there is an endpoint that has already been met, it would be unethical to subject further subjects in the study to placebo when they
could be getting the actual treatment. And so that’s exactly what happened. They met on April 27th. They looked at the data and they alerted the study coordinators that there was an endpoint that was met. So let’s go over the results. Here, they have exactly the same endpoints time to recovery and mortality. And the Remdesivir group, the time to recovery was 11 days. In the placebo, it was 15 days. And the p value h
ere was less than 0.001. Now if the p value is less than 0.05, then that means there is statistical significance. And that is the reason why the data safety monitoring board decided to alert the study coordinators that an endpoint have been met. Now when we look at mortality, the mortality on the Remdesivir group was 8.0%. A
nd in the placebo group, it was 11.6%. The p value here, however, did not reach statistical significance. But boy, it almost sure did, 0.059. Remember that they stopped the study early. If the study had gone to completion, this very well might have reached its historical significance. So given that, if the results were good enough to halt a study the questions
are is this enough to get people on Remdesivir and that’s exactly what they’re looking at right now. They are doing what is called an emergency use application or EUA. And the FDA is working with Gilead to see whether or not production can be ramped up to make enough Remdesivir to be used clinically in our nation’s hospitals. But the question is how effective is this medication Remdesivir. And so something
that’s a reasonable thing to look at is the number needed to treat to get an endpoint. In other words, how many people with COVID-19 would you need to treat with Remdesivir to save one person’s life? And the equation for that is the number needed to treat is equal to 1 over the absolute risk reduction. Now, the number needed to treat is a situation where if you have a low number, a number closer to one that shows that the intervention is very powerful..
For instance, somebody falling out of an airplane. If you were to give them just one parachute, how many people would you need to treat jumping out of an airplane with a parachute to save one person’s life? And the number there of course is one because it’s a very powerful and effective treatment and this number can go all the way up to 50, 60, 100 even past that. And depending on certain cardiovascular medications and the population that you’re talking about, these numbers can range anywhere from 10 to 20, to 30,
up to 50 and above. And so the absolute risk reduction here is 11.6% – 8%, which gives us 3.6%. But we need to put it numerically as 0.036. And when we do that, the number that we come up with is 28, so we would need to treat 28 people with COVID-19 that come into the hospital with Remdesivir to save one person’s
life. If you want to learn more about biostatistics, the number needed to treat there is more information and we have a video at MedCram.com. If you also want to learn more about Remdesivir and its mechanism of action, please check out COVID-19 update number 33.
We look forward to seeing you at the webinar where we will go over more comprehensively the treatment as it stands now for COVID-19. Thanks for joining us.



Add comment