Is COVID-19 a Disease of the Endothelium? (Lecture 63)
本文由‘中国推动’学者、江南大学药学院石漱新同学整理。
Welcome to another MedCram COVID-19 update.
Daily new cases, the United States are dwindling down.
However, daily deaths are not but they typically are delayed.
I wanted to take a look at a couple of other countries. Here, this is Brazil, daily new cases are going up to an all-time high.
As our daily deaths also in India total cases are starting to rise persistently.
As are the daily deaths in India. The cases in Iran have peaked about a month ago and have come down and have sort of plateaued at about a thousand daily cases. Deaths have done similar as well.
We have been looking at Finland back when we were talking about saunas and hydrothermal therapy. Those cases are also starting to come down as well. On the other hand, Sweden’s cases seem to be undulating but not really coming down in one particular direction.
In the last number of videos, we’ve been talking about the endothelial system as a potential target for the coronavirus and I want to show you what that looks like. This is the lumen of an artery and you can see here the white area is where the red blood cells in the blood moves and that endothelial lining coats every aspect of the wall of this artery. These are very thin cells and they control all sorts of things. And when they become
inflamed, certain things can happen. We’ll talk about those things.
Now, I’ve done a lot of research looking at particles and I think the picture is emerging of what it is that happens when SARS-Cov-2 infects the human being. I don’t have all the dots connected but there is a picture that’s emerging and many people have been affected and many people have died as a result of this. And I think you and society deserve a possible
explanation as to why we’re seeing the things that we’re seeing? Why aren’t ventilators doing what we thought they were going to do? Why are certain physicians seeing patients that are behaving like they’re at high altitude? Why do they have very compliant lungs when we would expect them to have very non-compliant lungs? Why are some young patients getting strokes, blood clots? Why are some patients getting better and even being extubated or taken off the ventilator only to succumb to cardiac arrest
or being discharged home and then coming back very sick later. I think a potential answer lies in the explanation that COVID-19 is an endothelial disease and it uses the lungs to get inside the body. And it no doubt causes disease in the lungs, but once it gets inside the body that’s where it causes its greatest damage. Now over the next series of videos, what I’d like to do is explain
exactly what I’m talking about using peer reviewed journals articles and research. But for you to understand a lot of what we’re talking about, we’re going to be using the language of oxidative stress. And for you to fully understand that, we’re going to have to go back to medical school and refresh on some basic things. So, please bear with me as we go over some basic ideas in terms of metabolism, oxidation, energy production
and some plain old biochemistry.
The first thing that we have to talk about is the batteries of your cell. These are called mitochondria. And they exist in the cell to produce energy. They also have their own DNA called mitochondrial DNA, which we’ll talk about later. Now notice in mitochondria, there is something called the matrix, which is this thing in the center, it’s a space in the center. And then there’s a space around the edge called the innermembrane space and that’s because it’s between
two membranes. That will become important later.
Here
, we’ve drawn it out schematically. Here’s the matrix in the middle and the innermembrane space around the outside. So when you eat food, there’s only three types of products that really matter and those are carbs, fats and proteins. Regardless of which one it is for now, we’ll simplify this a little bit. All of these things can eventually be broken down into two carbon units. This case it’s called acetyl-CoA. Then what happens is it
goes into cycle called the Krebs cycle. We’ll just call that circle K here. What happens is when it goes through that cycle, there’s a bunch of metabolism that occurs and out of that, comes basically electrons.
What’s important is that these electrons are tied up in something called NADH and FADH2.
These electrons are what we call reduced, which means that they love to be given up. You’ll hear me use that term reduced. Does it mean to go down and amplitude? It means the opposite of oxidized. What we mean by that is if you’ve got something like A and B and A has an electron and it gives that electron to B. Then what we say there is that B was reduced and A was oxidized.
In other words, when something loses an electron, it becomes oxidized and when something is gaining an electron, we say that it is reduced. So because these NADH and FADH2 are rich in electrons, they love to go out and reduce things. In other words, they become oxidized but they reduce something else in the process. So just keep that in mind.
And let’s back up a little bit and talk about why we’re here in the first place. Carbohydrates, fats and proteins are all fuel for our body. And when you put fuel into a car engine, what you’re trying to get out is angular momentum or rotational momentum of the wheel so it can spin and you can get energy. And that energy is being put to good use either by turning an alternator and getting electricity or turning a wheel and moving from one place to another.
Here, we obviously want something a little bit different. What the
endpoint is, is to get something called ATP that stands for adenosine triphosphate, which is basically an adenosine with three phosphate molecules on it. And what the body does to get energy is it just knocks off one of those phosphates and what you get is energy. And to get that energy back, you just take a bunch of energy to put that phosphate back on. Well, what are you putting it back on? You’re putting it back on ADP,
adenosine diphosphate. So when you go from adenosine diphosphate to adenosine triphosphate, you have to add energy. When you go from ATP down to ADP, you get energy. So this is the fuel for the body and that is the currency of energy in the body. Generally speaking, everything runs off of ATP.
So, how are we going to get from carbs and fats and proteins to ATP? This is what the mitochondria
for. And so carbs, fats and proteins are broken down into two carbon units. They go through this Krebs cycle. The Krebs cycle takes these things and basically metabolizes them into electrons, which can go around and reduce. So what happens now, these electrons then go here to this membrane where there are proteins in the membrane. So the electrons from Krebs cycle which are represented by NADH
, are ready to go to the innermembrane space to give up electrons. They are ready to give it up and there are proteins that are willing to accept it.
And when that happens, let’s blow this up a little bit to describe exactly what we’re seeing. So here we have the membrane. We have the innermembrane space and we have these electrons. And we have proteins in the membrane. So when these electrons come in, they come in at the very top
of the electron reduced scale, if you will, and so what they do is when they give up their electron, they give it down to a lower group and they lose some of that reduction. And in the process of doing that, as they go from one state of reduction down to a lower state of reduction, protons are pumped into the innermembrane space. And then it goes to another species, which is a little bit more oxidized,
and that pumps more protons into the innermembrane space. It keeps pumping and pumping until you get protons building up in this innermembrane space and so you have such a high concentration of protons in the innermembrane space that the pH in this innermembrane space is actually pretty low. And we’ll talk about what happens after that.
But finally what happens is this electron which started out here reduced and wanting to be reducing other
things becomes oxidized, finally it gets down to this point here where it’s no longer as reduced as it once was. And so the only thing now that it can reduce is the most oxidized molecule perhaps in the human body. In that case, it is oxygen itself. Oxygen will take this electron and turn eventually here, it takes that electron into water.
So you can see why oxygen is so important to the human body because without oxygen here, it could not accept the electron at the very end. And if it could not accept the electron at the very end, it would be like a traffic accident. Everything would back up and there would be no movement of electrons and if there was no moving electrons the protons would not move across into the innermembrane space. So why is it important to have protons in the innermembrane space?
Because the protons in their innermembrane space want to go back down into the matrix where there’s a low concentration of protons. And so they go through a channel and that channel is coupled so that as they pass through just like a dam on the Colorado River turns a turbine as these protons go through here. There is a coupling of ADP to, you guessed it, ATP. And we finally now have our currency.
So how do we go from carbs again,
fats and proteins to ATP? It gets metabolized down into two carbon units. The two carbon units goes into the Krebs cycle. The Krebs cycle turns it into electrons the electrons then go to the innermembrane space. Whereas they go down the electron transport chain to increasingly more oxidized carriers. It finally gets to the most oxidized electron acceptor which is oxygen, which then converts it into water. It reduces the oxygen to water. But in the process
of this electron transport chain, we get protons which are put into the innermembrane space. This builds up with a lot of concentration and then it comes down from the innermembrane space into the matrix coupling ADP into ATP. And now the body has energy. If on the other hand your cells don’t get enough oxygen, then the electron transport chain backs up, protons can no longer move into the innermembrane space. Therefore you don’t have a cascade coming down.
Therefore you don’t have ATP and the cell loses energy and dies.
So there are a couple points that I want to point out here. Number one, if you were to take a diet with quick metabolism of carbs, fats and proteins, you would find yourself with a surplus of electrons ready to move to the electron transport chain. Even though this is a regulated series of metabolism. You can see there a high carbohydrate, high fat diet will
quickly give you many electrons in The matrix of the mitochondria. So there is some good data that excessive high caloric intakes from either a high carbohydrate or high fat diet will cause more of these two carbon units to go into the mitochondrial Krebs cycle. And then what will come out of more electrons of course, which go to the electron transport chain. That’s number one. Number two is that oxygen is ready to accept any kind of electrons. It’s ready to accept electrons down here in the electron transport
chain. And if it’s ready to accept electrons here in the electron transport chain, it could certainly accept electrons anywhere along this path because more than any other species in the body, it loves electrons.
Fortunately, these electrons are kept in the form of NADH and FADH2, which is fairly tightly regulated. But sometimes if there’s too much NADH or FADH2, that can go awry.
So the next thing that we need to talk about is what happens if oxygen up here starts getting electrons outside of the electron transport chain, either because of misfortune or on purpose. Let’s talk about
out superoxides.
Okay. So this is where you really have to listen up and refer to this and slow it down if it’s too fast because this is really important here. We’re going to talk about oxygen.
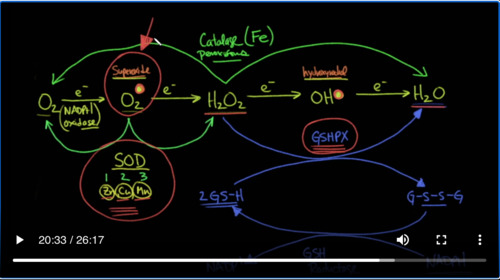
So we talked about oxygen being available to be the final electron acceptor in the electron transport chain. And so what we’re going to do is we’re going to go through this and show you what happens every time we add an electron. When we add an electron to oxygen, we get something called superoxide. And I’m going to put a big dot here to symbolize the fact that this has a single electron added to it. Whenever you have a single electron added in this case,
this becomes what we call a radical. So whenever we see this dot, this is a radical that means that this thing can be very reactive and it’s very dangerous and it can actually cause damage to your DNA and cause very bad inflammation. That’s important because the body actually uses this to kill bacteria and we’ll talk about that more. If we were to add another electron to that, we would get hydrogen peroxide. We know that hydrogen peroxide kills as well.
We add another electron to that. We’re going to get something called a hydroxy radical. Again, I’m going to put a big dot here. So you can see that is a dangerous species. If we add another electron to that, we finally get to water. So the ones that are dangerous are the ones with the hydroxy radical and the superoxide. I’m going to put the name of this here so you can see this is known as superoxide.
And this is a hydroxy radical.
This is bad for living things. So if you’ve got something living inside of you that you want to kill, the cells will make this neutrophils, etc. That’s exactly what they do. In fact, in the neutrophils, there’s something called NADPH oxidase. Anything that ends in an “ase” is an enzyme. So NADPH oxidase is an enzyme that oxidizes NADPH when its oxidizing NADPH. It’s
actually reducing oxygen to the superoxide radical. So this here is a bad player. This here is a bad player.
So when these things start to accumulate in your cells, your body has to have a defense mechanism to get rid of these things. Hydrogen peroxide is not a radical but it’s not exactly the best thing either. And so we have to come up with solutions. So one of the enzymes that’s really one of the great solutions to having superoxide
accumulate in your body is something called superoxide dismutase and that’s an important thing to understand. So superoxide dismutase takes the superoxide molecule, actually takes two of them, and converts it into an oxygen molecule and into a hydrogen peroxide molecule. So in other words, it gives one of these electrons to the other species and turns one of them into an oxygen and the other one into a hydrogen peroxide. So there’s three
types of superoxide dismutase. There’s type 1, there’s type 2 and there’s type 3. One of these is in the mitochondria. Another one is in the cytosol. And other one is in the extracellular matrix. And just so you’re aware, the elements, the metals that are required for this enzyme to work, very interestingly, are zinc, copper and when we’re talking about the mitochondria version of this manganese or Mn. So
these are very essential elements for superoxide dismutase to be able to work on this superoxide to get rid of it.
That still leaves us with this hydrogen peroxide. So the next enzyme which is very essential is something called glutathione peroxidase. We’ll abbreviate that as GSHPX. Glutathione peroxidase does exactly what you think it would do. It takes peroxide and it reduces it by giving it two electrons to water. But what does it get those two electrons from? Glutathione
can be in two forms, a oxidized form and a reduced form. And when in the reduced form, you have two equivalents of glutathione with an S and an hydrogen bonded to that sulfur group.
However, when it gets oxidized, it will look like this, GSSG. In other words, the hydrogens go and you have what we call a disulfide bond. How does that work? So here you have the reduced form in this redox reaction. The reduced form comes into here, glutathione peroxidase donates those two electrons to hydrogen peroxide, converting it and reducing it to harmless water. So how do we recharge our glutathione system? Well, we have to
reduce it back. The way we reduce it back is with NADPH, which has the two electrons and that converts that into NADP+. And that’s done through glutathione reductase. Why do we call that? Because we’re reducing glutathione. So here are the electrons are coming from NADPH. They go into this form which is the reduced form of glutathione. Then glutathione uses glutathione peroxidase to
reduce the peroxide back to harmless water.
Now. There’s another system that you should know about and that’s known as catalase.
Catalase can take hydrogen peroxide and convert it into water. Two molecules of hydrogen peroxide and converts the other one to oxygen. And that happens in the peroxisomes. Those are little organelles in the cell.
And the element that’s required for that to occur is iron.
So, what is it that I want you to know absolutely? I want you to know that superoxide is a very bad molecule. And it can cause a lot of damage. it can cause oxidative stress. And so the body has to come up with ways of solving that problem. One of the big ways is with superoxide dismutase. It requires zinc, copper and manganese. Another
way of getting rid of this is glutathione peroxidase. Glutathione peroxidase, then takes the resulting hydrogen peroxide and can turn it into water. If these systems are not working. What happens is you get a buildup of superoxide and that means a buildup of oxidative stress and that can lead to disease.
Now, sometimes in the literature, superoxide is sometimes referred to in general as ROS or reactive oxygen species. That will refer to superoxide, hydrogen peroxide and hydroxy radicals. So when you see these three, they are together known as reactive oxygen species and these are things that cause disease.
Okay. Let’s test our newfound knowledge and see if it’s going to
confer us any wisdom. Here’s an article that was published way back in 2008 titled “Angiotensin converting enzyme-2”, that’s the target of SARS-Cov-2, “confers endothelial protection and attenuates atherosclerosis”. So let’s go down into the article and see if we can make sense of this now.
Here’s a section called “ACE2 modulates Angiotensin II-induced ROS”, those are those reactive oxygen species,” production in endothelial cells.” So way back in 2008, we knew that. It says here “a major source of reactive oxygen species in endothelial cells is NADPH oxidase.”
Remember, that’s what we said right here, NADPH oxidase causes these reactive species, which in turn generates reactive oxygen species within endothelial cells Angiotensin II, remember Angiotensin II was the byproduct of ACE and this is a signaling molecule which causes vasoconstriction. This molecule is supposed to be metabolized by ACE2 to Angiotensin-(
1,7), which is a vasodilator. Let’s find out what they say here. “Angiotensin II-induced reactive oxygen species generation, as assessed by” this “dihydroethidium fluorescence was attenuated by ACE2.” That’s exactly what we would expect. Remember ACE2 is the protein that gets disabled when the virus binds it. “And this effect was attenuated by inhibition of Angiotensin-(1,7). We extend
these observations further by providing data that the effects of Angiotensin II to upregulate the expression of p22phox are attenuated by ACE2. In other words, ACE2, the Protein, that’s the target of the virus, is being downregulated and is no longer able to suppress Angiotensin-induction of reactive oxygen species. It goes on to say “these effects are also inhibited by A779”. That’s what they
use the experiments. They said here that “Angiotensin II-induced increase in MCP-1 and VCAM-1 in endothelial cells and are likewise attenuated by ACE2, and these effects are also blunted during coincubation with A779.” And just so you’re aware, A779 is a molecule that blocks the effect of Angiotensin-(1,7), the product of ACE2.
So here’s a summary of this paragraph, “these data suggest that ACE2”, remember this is the target of the virus, “in an Angiotensin-(1,7)–dependent fashion, functions to improve endothelial homeostasis, via a mechanism that may involve attenuation or reduction of NADPHox-induced ROS production.”
So let’s go back to our graph and see if we can modify it.
So what they’re saying here is that Angiotensin-(1,7) puts the brakes on NADPH oxidase.
But remember that Angiotensin-(1,7) is the product of Angiotensin II, and that is converted by ACE2. But SARS-Cov-2 destroys ACE2. And so what you have here is an inhibition of the inhibition, which means NADPH oxidase is allowed to go full bore,
and make these bad superoxide molecules. So the question is, is it possible that if somebody has a disease like cardiovascular disease or diabetes or obesity where superoxide dismutase is not working, and glutathione peroxidase is not working, and they’re already have one or two hits on their oxidative stress balance, and now all of a sudden, the
only thing that was keeping NADPH oxidase in check was Angiotensin-(1,7). And now with the virus, it knocks out the ACE, which is producing that Angiotensin-(1,7), and now you have free reign of NADPH oxidase. You’re going to get a huge buildup of superoxide in the system and that superoxide is going to cause endothelial damage and that maybe is what we’re seeing in COVID-19.
And now that you understand the language.
In the next upcoming lectures, we’re going to talk more about the studies that show that this may be actually what’s going on. Thanks for joining us and I look forward to seeing you on Thursday at the live webinar.



Add comment