COVID-19 and Oxidative Stress (Prevention & Risk Factors) (Lecture 65)
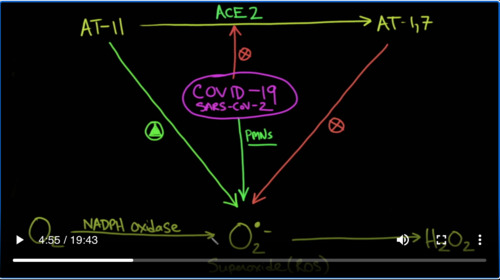
Welcome to another Medcram COVID-19 update and if you joined us live for our webinar, thanks for doing that. It’s also posted on our website and you can get the link here. I also want to let you know about the final AWR Symposium this Sunday that I’ll be doing a sleep talk in and we’ve left the link in the description below. I want to jump right into oxidative stress and where we left off before. So this is the picture that I want you to ingrain in your mind. Here we have oxygen on the left hand side and at every step we’re adding another electron. In other words. We’re reducing the oxygen will finally we get to the molecule water. These reactive oxygen species like superoxide right here and hydrogen peroxide and this hydroxy radical. These are the things that can cause oxidative stress and your body has in place mechanisms to get rid of it. It’s important here with superoxide dismutase both one, two and three that require certain elements like zinc, copper and manganese takes this superoxide molecule and converts it into a oxygen and a hydrogen peroxide. This hydrogen peroxide molecule is reduced further to water using glutathione peroxidase here and glutathione peroxidase is recharged by glutathione reductase and that is recharged by a number of antioxide chemicals and compounds that we can talk about. Also up here catalase which needs an iron cofactor can take hydrogen peroxide and convert it back into oxygen and into water. Now you should know that these antioxidants systems can be overrun and in certain types of conditions, like we’ve talked about diabetes and hypertension and elevated weight BMI. These can be overrun and so the question is what happens with COVID-19 infection. Because as we found out this is indelibly linked to a stew and angiotensin II, but I want you to focus on this superoxide molecule because we’re going to talk more about that now.
So I’m going to blow this up a little bit. We have oxygen here and it’s going to have an electron added to it.
And that’s what you get superoxide. And then if you add another electron to it, you’ll get hydrogen peroxide. And you should know that the enzyme here is NADPH oxidase. This is found in various parts of the body can be found in neutrophils as well. This here is known as superoxide.
And this is a reactive oxygen species. So what we know from the renin-angiotensin system is that angiotensin II is converted into Ang-(1-7) and that’s done by an enzyme called ACE2 and that’s a good thing because Ang-(1-7) decidedly blocks the production of superoxide. So it is blocking it and that’s a good thing that that’s happening because angiotensin II if left alone is actually a promoter of superoxide. Remember that superoxide is not a good species. It’s a reactive oxygen species and that causes inflammation and other bad things that we’ll talk about. So now enter COVID-19. Well, we know that covid-19 or specifically the SARS–CoV-2 virus binds with a stew as its receptor and basically in activates it. So COVID-19 is going to shut down ACE2 and you can see the complications of that because COVID-19 is now going to prevent the production of Ang-(1-7) which is helping us keep superoxide in check. COVID-19 also because it blocks ACE2 is going to increase the amount of angiotensin II which is getting increased in the amount of superoxide. Just for clarity, I will put the name of the virus. But the other thing that covid-19 does is it causes inflammation and specifically it recruits neutrophils or PMNs, and PMNs like to use a very similar enzyme NADPH oxidase to stimulate the production of superoxides.
And this is what we feel is going on with SARS-CoV2 COVID-19. Okay. Let’s see if we can find some evidence for this. Where do we see that angiotensin II can cause problems with reactive oxygen species. Well, here’s a review article titled “Oxidative stress in atherogenesis and arterial thrombosis: the disconnect between cellular studies and clinical outcomes”. And if you notice it says here “Evidence for a critical role for NAD(P)H oxidase–derived oxidatived stress in a atherosclerosis has come from both cell culture studies and animal models of atherosclerosis. NADPH oxidase and there’s our friend superoxide production is increased in vascular cells by a variety of agonists relevant to the pathogenesis including—— there it is——angiotensin 2
But we don’t have to stop here. Here’s another article titled “ Oxidative stress–mediated effects of angiotensin II in the cardiovascular system”.
And here’s an excerpt that looks like they just read what we wrote on the screen “Oxidative stress describes an imbalance state while the production of ROS, including superoxide, hydrogen peroxide and hydroxyl radicals, exceeds antioxidant defenses”. There are several enzyme systems contributing to the formation of ROS including NADPH oxidase, xanthine oxidase and mitochondrial electron leakage from electron transport chain. Remember we talked about that last video.
ROS or reactive oxygen species are normally generated as a natural byproduct of oxygen metabolism and play important roles in cell signaling. However, reactive oxygen species levels can be increased dramatically under oxidative stress conditions such as heart failure, ischemia and aging. And then it goes on and talks about how angiotensin II stimulates in vitro and in vivo the increase in reactive oxygen species.
So I think we’ve made the case that angiotensin II definitely stimulates the formation of reactive oxygen species. What about PMN in COVID-19 and SARS-CoV-2 infection. Does this cause pmn to secrete superoxide? Let’s see. Well, here’s an article that was published and we’ve reviewed this before. This article looks over the similarities between the first SARS virus, the MERS virus and the current COVID-19 disease.
Here under the heading“Innate immune responses to SARS-CoV-2 infection gaining insight from strategies used by SARS–CoV and MERS–CoV”. They say only currently limited information is available on the host and immune status of SARS–CoV-2 infected patients. In one report where 99 cases in Wuhan were investigated, increased total neutrophils(38%), total lymphocytes reduce 35%, increase serum IL-6(52%), and increased c-reactive protein(84%) were observed. In a separate report also from Wuhan, it revealed that 41 patients had increased total neutrophils, decreased total lymphocytes in patients in the ICU vs. non-ICU. So these were the more severe patients.
And of course, it’s well–known as it is here in this paper that neutrophils cause reactive oxygen species to be generated by NADPH oxidase will put these links in the description below as well.
So I think we’ve shown good evidence that this arm is also a reality. What about Ang-(1-7) and the fact that it may block or suppress superoxide. What’s the evidence for that?
Well, here’s a paper that was published in 2008. They knew them at angiotensin converting enzyme-2 conferred endothelial protection and attenuated atherosclerosis. Basically, you can see the results here in the last sentence. They say here that these data indicate that ACE2 in an Ang-(1-7)-dependent fashion, functions to improve endothelial homeostasis via mechanism that may involve attenuation of you guessed it NADPHox-induced reactive oxygen species production. ACE2-based treatment approaches maybe a novel approach to limit aberrant vascular responses and atherombosis. Here’s another paper that was published about five years ago titled “ACE2 and Ang-(1-7) protect and endothelial cell function and prevent early atherosclerosis by inhibiting inflammatory response”.
So they hypothesize that ACE2 overexpression and Ang-(1–7) may protect endothelial cell function by counterregulation of angiotensin II signaling and inhibition of inflammatory response, and they were right. Their conclusion was that ACE2 and Ang-(1–7) significantly inhibited atherosclerosis lesion formation via protection of endothelial function and inhibition of inflammatory response.
And this one’s probably the icing on the cake. This was published just this year in January before we even really have a clear understanding of coronavirus. You see patients who have preeclampsia have tremendous amount of vasoconstriction and they believe this is mediated by angiotensin II. So these are pregnant women who are about to give birth or near the stages of giving birth and they have high protein levels, they have high blood pressure and I believe that this is related to angiotensin II and so they decided to give local Ang-(1–7) administration to see if that would help with preeclampsia. They say here despite remission of clinical symptoms postpartum, women who have had preeclampsia demonstrate microvascular endothelial dysfunction mediated in part by increased sensitivity to angiotensin II. They say that Ang-(1-7) is an endogenous inhibitor of the actions of and angiotensin II and plausible druggable target in women who had preeclampsia. So what did they find that Ang-(1-7) increased endothelium–dependent vasodilation via NO synthase–mediated pathways and attenuated angiotensin II–mediated constriction in women who have had preeclampsia, suggesting that Ang-(1-7) may be a viable therapeutic target for improved microvascular function in women who have had preeclamptic pregnancy
So again, I believe there is good evidence for the schematic that we have drawn here regarding superoxide and this reactive oxygen species. Now so far things seem to be lining up, but it could all be just coincidence. Sometimes science is funny that way, but what’s interesting is if you look at this article by JAMA that was published just about a week or so ago looking at 5700 patients hospitalized with COVID-19 in the New York City area. What I’ve got listed here is the number of patients that were seeing that had for instance hypertension (57%), coronary artery disease (11%), congestive heart failure (7%). And if you’re not aware, these numbers are much higher than they are in the general community. How about BMI or body mass(>30), 42% of the patients that were admitted to the hospital had a BMI greater than 30. What about those that were admitted that had asthma it was about 9%. What about those who had COPD( 5%), obstructive sleep apnea(3%), and that’s about the same as it is in the population. What I did here was I looked up as best as I could in the community in New York City. What the general population of patients have that had hypertension, it was about 29 %. And what is the general population in the New York City area that has a BMI greater than 30 it was about 28%. And then I look to see how much COPD there was in New York City and it was about 5%. This is not a standard rigorous epidemiological study, but I wanted to get a sense and it’s interesting. Because when this virus was coming to the United States, a lot of Our advice even as a pulmonologist was to talk to our COPD patients and to talk to her asthmatic patients and say “look, this is a bad virus, you really want to be the one to stay home. This is going to affect our patients with lung disease because we thought this was a really bad disease for the lung”. And in a way it is, but it seems very clear here that if there’s 5% incidence of COPD in the population and 5% of them in the people that are being admitted to the hospital. It would make sense to say that COPD is not a really big risk factor. On the other hand, when there’s only 29 % of the people in New York City that have hypertension, and 57% of the people in the hospital with covid have hypertension we’re starting to see that this is hitting people with cardiovascular disease and it’s cardiovascular disease and obesity that we know has a problem with oxidative stress.
And that brings us to this 24 page review article with over 300 references titled “Nutrients and Oxidative stress: Friend or Foe?” And it basically is talking about what we see here with patients who have obesity and coronary disease, hypertension, cardiovascular disease. It by the way is an excellent article, it reviews oxidative stress. It talks about the different diseases and molecular connectivity of oxidative stress–induced diseases. It talks about diabetes, cardiovascular disease, cancer etc. It also talks about diet which is very important and the importance of things like whole grains in reducing oxidative stress.
And it finally ends on this picture. Of these are the patients here on the left that seem to be more represented in the patients who are being admitted to the hospital with COVID-19. Clearly, it states that oxidative stress increases your chances of going to the left where as reducing oxidative stress is moving you to the healthier stage here on the right hand side.
So is it possible perhaps then when we look at our schematic and we see here that a COVID-19 infection or disease state is going to certainly tip the balance in favor of oxidative stress. That the reason why some people do better and some people don’t may have to do with the current state of the oxidative stress in their body at that time.
And clearly here. There is a difference between the man on the right and the man on the left in terms of where oxidative stress is in that particular patient.
But your question now at this point must be what does this have to do with thrombosis? How does oxidative stress lead to thrombosis? Well, there are a number of articles that connect oxidative stress with endothelial cell dysfunction and thrombosis and that’s not a far stretch. And the reason is is because one of the primary purposes of the endothelial cell is to prevent thrombosis. And so when it is not working, thrombosis is a natural consequence. Remember what we said about Ang-(1–7), we set here that Ang-(1–7) works through a nitric oxide(NO) potentially intermediate.
Here we read that endothelial dysfunction is the earliest phenotypic change in the vasculature following exposure to atherothrombotic risk factors. And the theory of dysfunction is associated with decreased synthesist and increase oxidative inactivation of nitric oxide(NO). Critical antioxidant enzymes just like the ones we told you about——essential for eliminating reactive oxygen species that can inactivate nitric oxide. So nitric oxide is good.
Include and here we go. Superoxide dismutases, glutathione peroxidases and catalase as well as another one which we haven’t talked about glucose-6-phosphate dehydrogenase. Deficiency is of these enzymes increase oxidative stress and nitric oxide in activation and, as such, can either lead to and ethereal dysfunction or account for the underlying mechanism of endothelial dysfunction associated with a given atherothrombotic risk factor. Selected antioxidants improve intracellular redox state and reverse and the ethereal dysfunction by improving the bioavailability of NO. These observations provide mechanistic insights into the molecular basis of endothelial dysfunction in vascular disease and its treatment. This was published almost 20 years ago.
And here’s another article titled “Oxidative stress in endothelial cell dysfunction and thrombosis”.
Notice in this article. It says that endothelial dysfunction is the earliest phenotypical change in the vasculature and that all these things: hypertension, hypercholesterolemia, cigarette smoking, diabetes mellitus, and hyperhomocysteinemia all induce this in the absence of established atherothrombotic disease. They also mentioned the key molecular mediator NO.
And so what we’re starting to see is that these players that we put here in our schematic are certainly tipping the scale in favor of pro-oxidant of oxidative stress. And in our bigger scheme, if these systems which are supposed to keep things reduced are not working, either because of dysfunction or because of abuse. These are the patients that are not going to do well when they are tested with COVID-19
So this presents itself many points of intervention, and we still have a lot to cover on this topic. But for now, we’re going to take a break. Have a great weekend and we’ll see you next week. Thanks for joining us.



Add comment