Welcome to another MedCram COVID-19 update. A quarter of a million people confirmed infected worldwide. There were over 10,000 deaths and 86,000 total recovered. If we look on the Worldometer chart of individual countries, Italy has by far the most active cases here at 33,000 and has the most number of total cases per million population.
欢迎来到MedCram COVID-19的另一个更新。全世界有四分之一的人确认感染。有10,000多人死亡,总计86,000人康复。如果我们查看各个国家/地区的Worldometer图表,则到目前为止,意大利的病例最活跃,为33,000,每百万人口中的病例总数最多。
The United States has jumped up to about 14,000 total cases. In terms of total cases per million, we’re at 43; that maybe because of low testing although that seems to be getting better here slowly.
美国的病例总数已跃升至约14,000。就每百万例总数而言,我们为43;那也许是因为测试不足,尽管在这里似乎慢慢变得更好了。
I wanted to show you something that I received; it’s called the Handbook of COVID-19 Prevention and Treatment, and this is actually published by Professor Tingbo Liang, and he goes through various things. He has a forward at the beginning, and the content is anything from staff management to imaging findings on COVID-19 patients, all the way to ECMO support for COVID-19 patients. What’s going on with this, I think, is very important, is just the experience and the information for us because they’ve gone through this already. I will leave a link in the description below.
我想向您展示我收到的东西;它叫做COVID-19预防和治疗手册,实际上是由梁廷博教授出版的,他经历了许多事情。他一开始就很有前途,内容涉及从员工管理到对COVID-19患者的影像学发现,一直到ECMO对COVID-19患者的支持。我认为这很重要,因为对于他们来说,这只是经验和信息,因为他们已经经历了这一过程。我将在下面的说明中保留一个链接。
Other big news, hydroxychloroquine was fast-tracked by the FDA yesterday, March 19th. The data for this medication is not the rigorous data that the FDA would usually want to have, which is a pretty extensive randomized controlled trial with placebo Etc. That still has not been done, but because of the situation and because there was some early data out of France that seemed to show that it was positive, and of course we here at MedCram have been looking at this drug for some time, saying that it was a promising medication at least on the in vitro side of things, we can see why that happened.
其他重大新闻是,羟氯喹于3月19日被FDA快速追踪。该药物的数据并非FDA通常想要的严格数据,这是一项使用安慰剂Etc进行的相当广泛的随机对照试验,目前尚未完成,但由于情况而定,因为有一些早期数据在法国,这似乎表明它是阳性的。当然,我们在MedCram的实验室已经研究了这种药物已有一段时间了,说它至少在体外方面是一种很有前途的药物,我们可以明白为什么那事发生了。
This hydroxychloroquine is also known as Plaquenil. It’s a medication that’s used in patients with lupus. For instance, I called around specifically today to different pharmacies, and there was none available where there was last week; there was absolutely none available today. Of course, this brings up issues about drug availability and the ability for any of our resources that we have, whether it’s personnel, whether it’s physicians, health care providers in general, beds, ventilators, medications, do we have enough to meet the surge with this many people getting sick all at the same time?
该羟基氯喹也称为普拉克尼尔。这是狼疮患者使用的药物。例如,我今天特别拜访了不同的药店,而上周没有人在那儿。今天绝对没有空。当然,这带来了关于药物可用性和我们所拥有的任何资源的能力的问题,无论是人员,医生,一般的医疗保健人员,床,呼吸机,药物,我们是否有足够的能力来应对这种激增有这么多人同时生病?
There’s a news article in the Duluth News Tribune that was published a couple of days ago, and it’s looking at the fact that there are a lot of health care professionals that are contracting COVID-19. Is there a way that we can test to see whether Hydroxychloroquine might prevent that? We see here that David Boulware is actually starting a study to see whether or not we can reduce the number who get sick by 50% of these health care providers. In this study, the volunteers are going to get either the medication or placebo.
几天前在Duluth新闻论坛上刊登了一篇新闻文章,它关注的事实是,有很多医疗保健专业人员正在感染COVID-19。有没有一种方法可以测试一下羟氯喹是否可以防止这种情况?我们在这里看到David Boulware实际上正在开始一项研究,以查看我们是否可以将这些医疗保健提供者的患病人数减少50%。在这项研究中,志愿者分成药物或安慰治疗。
Also, news last night, Governor Newsom of California locked down the entire state. Of course, that’s relevant to where we are here as I live in Southern California, with his prediction being that 56% of the state residents will be infected with coronavirus in the next eight weeks.
同样,昨晚有消息称,加利福尼亚州州长纽瑟姆(Newsom)封锁了整个州。当然,这与我在南加州生活时所处的位置有关,他的预测是,在接下来的八周中,将有56%的州居民感染冠状病毒。
Of course, the big news a couple of days ago was this new medication called Favipiravir which may be effective in coronavirus. Let’s go over the results of that.
当然,几天前的大新闻是这种称为Favipiravir的新药可能对冠状病毒有效。让我们来看一下结果。
So the trial looked at the intervention group for Favipiravir and Lopinavir and Ritonavir. There were 35 people in the treatment group and 45 people in the control group. Interestingly, the New England Journal of Medicine article that we talked about yesterday showed that this was basically no different than placebo. Number one, they looked at the clearance of the virus in terms of days, then how long it took for the fever to go down for it? How many was the fever gone within two days? They want to know whether or not the chest x-ray improved, and they wanted to know if there were any adverse reactions.
因此,该试验着眼于Favipiravir,Lopinavir和Ritonavir的干预组。治疗组有35人,对照组有45人。有趣的是,我们昨天谈论的《新英格兰医学杂志》文章显示,这与安慰剂基本没有区别。第一,他们看了几天的病毒清除率,那么发烧持续多长时间呢?两天内发烧了多少?他们想知道胸部X光片是否改善,他们想知道是否有不良反应。
These were statistically significant. In terms of viral clearance and days, it took 4 days in the treatment group and 11 days in the control group, so it is statistically significant that was p-value of less than 0.001. In terms of fever gone at 2 days, that was seen in 72% of the treatment group but only 26% of the control group; in terms of chest x-ray improvement, that was seen in 91% of the treatment group and only 62% of the control group, and interestingly in terms of adverse reactions, that was only seen in 11.4%, but in the control group adverse reactions were seen in about 56%.
这些具有统计学意义。就病毒清除率和天数而言,治疗组为4天,对照组为11天,因此p值小于0.001具有统计学意义。就第2天发烧而言,这在治疗组中占72%,而在对照组中仅为26%。就胸部X光改善而言,在91%的治疗组中可见,仅在对照组的62%中,有趣的是,在不良反应方面,仅11.4%可见,但在对照组中约有56%的人出现了反应。
Okay. Well, we’ll be back soon for our next update. Thanks for joining us.
好的。好吧,我们很快就会回来进行下一次更新。感谢您加入我们。

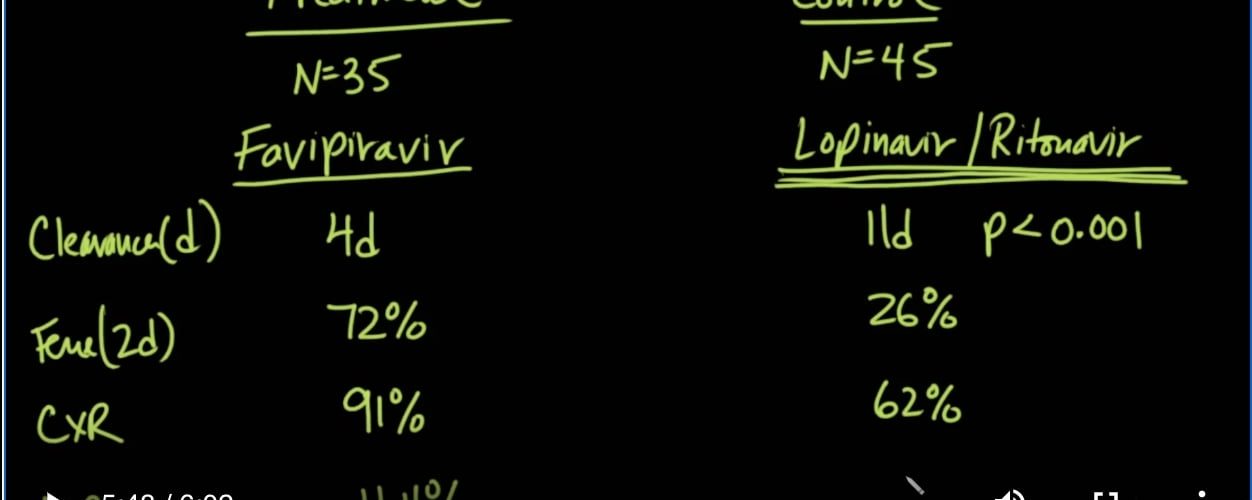


Add comment