Update 87: More on Dexamethasone; Do COVID-19 antibodies last?
本文由‘中国推动’学者、江南大学药学院石漱新同学编辑整理。
MedCram.com. Welcome to another MedCram COVID-19 update.
We are approaching 9 million infected worldwide and half a million deaths worldwide. Here in the United States, 2.3 million cases with over 120 thousand deaths, with daily new cases starting to rise. But for the 8th week in a row, death rates continued to drop. And of course, the big news in the last number of days is a new medication potentially to the regimen of COVID-19, which is dexamethasone.
We recently heard from Dr Peter Horby, the co-investigator of the recovery trial out of Oxford, discussing exactly what happened during that trial, and what happened with patients on dexamethasone versus patients not on dexamethasone in terms of recovery, in terms of mortality. And we’ll link to this video as well in the description below.
This of course has set off a huge demand for dexamethasone as published here in Science. They talk about where they really don’t have any evidence that it’s going to run out. But there is some evidence that people are trying to get their hands on dexamethasone, which of course is only one of many different types of corticosteroids that are available. The key being, of course, that the recovery trial used specifically dexamethasone either orally or intravenously in the trial at 6mg once daily.
But it is pointed out in this New England Journal of Medicine journal watch, Dr Paul Sax talks about this is such great news, what has been the response to this great news? He says here in his tweet, “very welcome news, dex is cheap, widely available! Demonstrates the power of randomized controlled trials versus observational studies, which were conflicting; How will the numerous ongoing studies of immunomodulators be modified? And treatment guidelines – act now
or wait for more information?” And what he says is really interesting. He says here, “But my assumption was that the clinical practice would change quickly, awaiting the updating of guidelines. After all, this is what we’ve been waiting for – data from a randomized trial, demonstrating a clear benefit. Even better, it’s readily available, inexpensive strategy – a course of corticosteroids – familiar to us all.”
Well, the response he got bloom away. He says, ” I confess the responses to my post in comments elsewhere surprise me. Lots of skepticism. Wow.” And he goes on to show that the comments fell into several subcategories and here was one of them, he said that there were some people that responded and said, “let’s wait for the study to be peer-reviewed and published in an established medical journal before changing clinical practice.” And of course, that sounds reasonable, but there are a couple things that are different
about this. Number one, it’s not being released by a drug company. It’s being released by a study design that was funded publicly by a government, not to unlike a data safety monitoring board that makes decisions that says, “hey, maybe this is not a good idea to continue to study because we’re seeing a difference and it would be unethical to continue this for those people getting placebo.”
Others responded this way, “why are we getting critical information via a press release? I’m inherently distrustful. A press release doesn’t represent actual data.” Again, he says, ”consider this isn’t a press release by a giant company, citing a minor change in an inflammatory cytokine or quality of life metric and an open label study. It’s a respected clinical trials group funded by the government of Great Britain and the reporting a survival benefit from their clinical trial.
” Then he goes on to say that while the rest of the world was trying to throw any medication that ended in mab at COVID-19. They were actually enrolling patients in randomized controlled trials. He also says, “some people said here we go again, haven’t we been burned already multiple times with research on COVID-19 only later to have this information question or retracted. He says true but those things involve much weaker levels of evidence. This is a randomized controlled trial showing
survival benefit.
Other people were saying, “I need more details about the study. What were the primary endpoints, the specifics of the intervention? What were the patient characteristics of those enrolled? Did some subgroups benefit more than others? What were the toxicities?” Well as he points out the good news is that we actually have the full protocol available for review.
And here it is and we’ll post this in the description below so you can read it for yourself. Of course, this is all of the drug trials
in the entire recovery trial. So, be aware of that when you go through the protocol. So, in the end, what do we make of this? We won’t know until the actual data comes out and it gets its vetting and its peer review, which of course needs to happen.
A couple things to consider. In the trial, they used it for 10 days. That’s way too short to see things like osteoporosis, cataracts, diabetes, new onset diabetes. But it’s certainly a long enough course to see
immunosuppression in patients with concomitant infections. And that’s been the concern up to this point. Because this medication takes out the rug from the immune system, you really want to make sure that the immune system doesn’t have its hands filled doing something else, instead of just trying to battle COVID-19. And that is the inherent risk in starting dexamethasone. It’s a small risk, but one nevertheless.
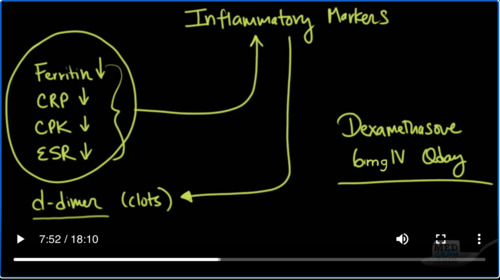
Now, I’ve just spent the last three weeks in a row taking care of COVID-19 patients. And I’m starting to get a sense what the natural history is of these patients, typically come in and there’s a number of days where they get worse. And their inflammatory markers start to go up.
Inflammatory markers, for instance, like ferritin,
CRP or C-reactive protein, CPK, which is a byproduct of muscle breakdown, ESR, which is the erythrocyte sedimentation rate. These are all signs of inflammation. So, the else that we also monitor is d-dimer levels and d-dimers may also tell you about clots, which may be an effect of that inflammatory process causing blood clots to occur in the lung. We know
based on the evidence from autopsies that COVID-19 patients have 9 times more blood clot burden in autopsy samples then for instance in influenza. So, the general trend is I see these things going up early in the course, and then one by one, they start to come down. What I’m seeing now in the last week because we’ve all been using dexamethasone at our hospital, 6mg IV Qday.
What I’ve seen is a reduction in these markers more quickly than my experience before we started dexamethasone.
The question is of course, is this a real finding that I’m seeing or is it just anecdotal evidence? That’s number one. And number two, is it just reducing the surrogate markers of inflammation but not affecting the actual outcome and natural history of COVID-19. In some of these patients, I’m starting to see their oxygen requirements come down. Of course, I saw this happening before we started dexamethasone. Is it just a recall bias? I don’t know for sure at this point.
I know at the hospitals that I work at, we were not routinely using dexamethasone before this announcement. And now we are. But I know for a fact that there are some hospitals and physicians out there that were using corticosteroids long before we started using it. And there are some hospitals even now that are not using dexamethasone or corticosteroids and they are waiting for the final data to come out.
So, it’ll be interesting to see here at least in the United States that as the daily new cases go up.
What will happen to daily deaths? Especially given the fact that there will be a significant number of hospitals and health care providers that will be using steroids now in COVID-19.
I wanted to switch gears a little bit and talk about the vaccine tracker that we have been showing. As you know, it’s giving us updates on a regular basis about the candidates, the sponsors, the trial phases, the institution and the funding for the various different types of vaccines.
In this vein, I wanted to highlight a paper that was recently published in Nature Medicine a few days ago, coming out of China, looking at the difference in antibody responses and longevity in comparison to those that had asymptomatic disease with COVID-19 and those that were symptomatic. And of course, we’ll post a link in the description below. And this article is titled “Clinical and Immunological assessment of asymptomatic SARS-CoV-2
infections”.
So, these were all hospitalized patients. In the thing you have to understand is in China, they were actually having patients go to these hospitals, even though they were asymptomatic. So, they were able to study asymptomatic COVID-19 patients versus symptomatic. And it was a relatively small study. They looked at 37 asymptomatic COVID-19 patients. And what they found is that in terms of viral shedding.
The average was 19 days for asymptomatic, which is more than two weeks. And the symptomatic on average was 14 days. So, the asymptomatic actually shed the virus for a longer period of time and that was statistically significant. When they looked at the immunoglobulin levels, the amount was 3.4 in the asymptomatic, whereas the symptomatic had a much higher immunoglobulin level
at 20.5.
Furthermore, when they look down the line after a period of time, they found that 40% of the asymptomatic COVID-19 patients were seronegative. What does this mean?
There was no antibodies that they could find any more against the coronavirus in these asymptomatic patients. Whereas in the symptomatic, that number was not 40% It was only 12.9% seronegative.
Now, just because they didn’t have antibodies doesn’t necessarily mean that they couldn’t mount an immune response. Remember that there are T cells. There are memory T cells, which can quickly reactivate in the presence of a reinfection. But what this shows is that these antibodies may not be hanging around for a long period of time.
Here you can see a graph of the positive rate of viral RNA versus days in terms of duration of viral shedding. And you can see clearly that the asymptomatic cases shed the virus for longer than the symptomatic cases.
I thought this part was interesting here in the discussion of the study. “Recently, several studies characterizing adaptive immune responses to SARS-CoV-2 infection have reported that most COVID-19 convalescent individuals”, that means individuals that have recovered from the virus, “have detectable neutralizing antibodies, which correlate with the numbers of virus specific T cells.” In this study, we observed that IgG levels”, those are at the antibody levels, “and neutralizing
antibodies in a high proportion of individuals who recovered from SARS-CoV-2 infection start to decrease within two to three months after infection. In another analysis of the dynamics of neutralizing antibody titers in 8 convalescent patients with COVID-19, four patients showed decreased neutralizing antibodies approximately six to seven weeks after illness onset. The one mathematical model also suggests a short duration of immunity
after SARS-CoV-2 infection. Together, these data might indicate the risks of using COVID-19 immunity passports’ and support the prolongation of public health interventions, including social distancing, hygiene, isolation of high-risk groups and widespread testing.” Again, a lot of this is conjecture and we don’t know until we actually have patients that have had the virus, that’ve had the antibodies, test negative for the antibodies and then see whether or not they can be infected again.
And finally, I wanted to talk to you about what they’re doing in the United Kingdom in terms of vitamin D research. Of course, the United Kingdom is a little bit higher in latitude than the United States. And there’s been some good data coming out of there that has shown not only that vitamin D is associated with viral chest infections, but it’s also associated with outcomes in COVID-19. And also there’s been a disproportionate amount of vitamin D deficiency in people of color in that country as well.
There’s been a lot of good research and data on vitamin D, including a meta analysis that was done number of years ago in the British Medical Journal. And we have MedCram.com where one of the first to make that apparent and it’s connection with COVID-19 back in the early part of March. And this particular study is actually looking at people to sign up to get more information about levels and their susceptibility to COVID-19. And we will put
a link to this article. And this study called COPVIDENCE UK, your data, fighting coronavirus. So, if you live in the UK, this is something to definitely consider signing up for.
One more thing that we were talking about very early on in the COVID-19 course back on January 28th in our video, how coronavirus kills, were three things that improve ARDS and one of those things that we talked about which we believe would be something very important
to do in coronavirus patients was prone positioning. And this has been expanded to awake non-intubated patients with COVID-19 hypoxic respiratory failure. This article was recently published in the JAMA network on June 17th 2020. And basically what they did here at Columbia University step-down unit was they asked 88 patients to sign up for the study and they looked to see if they put these patients in the prone position, basically
laying on their stomach, whether or not it would improve oxygen saturation to above 95% after one hour of initiation of the prone position. Let’s read what they say here in the discussion, “in this small single-center cohort study, we found that the use of prone position for awake, spontaneously breathing patients with COVID-19 severe hypoxemic respiratory failure was associated with improved oxygenation. In addition,
patients with a SpO2”, that’s a finger pulse oximetry, “of 95% or greater after one hour of the prone position was associated with a lower rate of intubation. Limitations of our study are the lack of control group, small sample size.” And they say that randomized controlled trials, of course, always are needed to establish whether the improve oxygenation after use of the prone position actually improves survival.
So, I can tell you from my own experience that we’ve had patients in the COVID
unit that looked great, they were just severely hypoxic. And as I saw them getting worse and worse, we went into the rooms and really educated them because they were awake, alert, communicative about how important it was for them to lay on their belly. In fact, we help them do that and get into positions that were comfortable enough for them to stay in that position for hours on end. They were of course were very motivated to do that and we saw dramatic changes. I mean, I’ve seen the partial pressures of oxygen go from 60 to
over 200 on the same requirement of oxygen. I’ve never seen anything like that before. I’ve seen it in these kind of patients.
So, as we look at this from a global perspective, I am cautiously optimistic that with the combined use of remdesivir, in addition to dexamethasone and awake proning with supplementation of some of these things that have very low risk factors, very low risks, things like vitamin D, vitamin C, zinc, in some cases anticoagulation and if necessary, convalescent plasma, which is still experimental.
That we may now for the first time be going down a road that shows that we can reduce mortality substantially in these patients. And I’m very excited to see whether or not that is going to pan out.
The reduction in daily deaths that we’re seeing in the United States could be due to a number of factors as we talked about. It could be a different population that’s becoming infected now, maybe it’s a younger population. It could be down that we’re in summer. Now, it was since we’ve had the longest day of the year, people are getting more
vitamin D. It could be that our medical care is getting better. It could also be that the virus has weakening. We also have to realize, of course, that this daily death graph is behind by a couple of weeks the actual new cases because there is a natural history of how this virus affects the human body and it delays by a couple of weeks. Nevertheless, we are seeing a substantial decrease in the death rate and it’s been a continuous one now for about seven or eight weeks in a row.
I hope that this continues and of course we will continue to update you. Thanks for joining us.



Add comment