Anticoagulation; Can Mechanical Ventilation Make COVID-19 Worse (Lecture 53)
Welcome to another COVID-19 MedCram update taking a quick look at the numbers. We are at 1.6 million confirmed and 97,000 deaths worldwide. Currently the hot spot in terms of new cases is the United States and Spain followed by Germany. If we look at where most of the deaths are coming from in the world. It’s United States and France as the top two countries at this point.
And in terms of the United States New York, of course it’s the epicenter of that and it looks as though they are projecting in terms of bed availability, a shortage as we go here into the peak. We look at the number of deaths, they have spiked over the last couple of days but are predicted to start to fall in the very near future. And of course, these are based on projections from a mathematical model out of the University of Washington.
I wanted to talk about the ventilation issues that we’re dealing with in terms of COVID-19. And there was a very interesting paper that came out recently by Dr.Gattinoni who is a physician in Italy and what he’s saying here is that there are two different types of diseases that he seeing with COVID-19 in terms of lung problems. He’s calling it a Type L and a Type H and he writes a letter here that we will link to in the description below and the best way that I can describe it is in order for your lungs to breathe and to oxygenate effectively. You have to have the right amount of air going into the lungs and the right amount of blood circulating to the lungs. If there is too much air and not enough circulation or too much circulation and not enough air you’re not going to get the optimal amount of oxygenation in the blood. Typically speaking, when patients come in with an pneumonia from a viral infection what we’ve seen in the past almost exclusively has been a problem with the amount of are getting into the lung, not a problem with the amount of blood getting to the lung. And that’s what we all prepared for. That was a term called ARDS acute respiratory distress syndrome. This is when the lungs fill up with fluid, there’s not enough air getting down into the tiny air pockets called alveoli. The way to overcome that is to put patients on ventilators because ventilators are very good at pushing that water out of the way and getting air down there so that oxygen can diffuse into the blood that is flowing around the lungs. What Dr.Gattinoni is suggesting here is that perhaps there’s also another type of lung problem that we’re seeing in COVID-19 and that is not a problem with air getting into the lungs, but a problem with blood getting to the lungs potentially and that’s what he’s referring to here in this Type L——type of pneumonia and that has to do with low ventilation to perfusion.
He’s saying here that this may be explained by a loss of regulation of perfusion and by a loss of hypoxic vasoconstriction. Accordingly, at this stage, the pulmonary artery pressure should be near normal. These people have nearly normal compliance of their lungs which means that their lungs are able to move without a problem which means there’s not a lot of water in there. Now it can very well be that these are how the patients with COVID-19 are presenting initially. But as more and more fluid starts to accumulate in the lungs, they start to look more and more like the original type of lung disease that we would expect to see and that would be the Type H, and that would be a lung full of fluid which would make itself amenable to ventilation on a ventilator. There is shunting of blood past these areas where they’re not getting ventilated and so the lungs are full of water and have a high lung weight and because of that if you put enough pressure down into the lungs you can actually pop those alveoli open nicely and ventilate them. Dr. Gattinoni says this Type H pattern which was what we were all expecting, account for just 20% to 30% of the patients in their series that fulfills ARDS criteria.
Dr. Gattinoni in his letter goes on to talk about how you may want to try to buy time in these Type L patients who have very good lung compliance to see if they will get better without having to be intubated and put on the ventilator. However, if you do have to intubate these patients who are Type L. They say they can be ventilated with volumes greater than 6 ml/kg, and that remember is what we typically ventilate patients with Type H, the ones who have ARDS, the ones with low lung compliance, these high compliance results means that you can ventilate them with higher volumes and because of that you might be able to reduce the PEEP down to a lower level than you would expect in patients with Type H. As he says here that Type H patients should be treated as severe ARDS and have higher people levels, that’s the pressure that is left in the circuit at the end of exhalation, if compatible with hemodynamics prone positioning and extracorporeal support. So Dr. Gattinoni concludes by saying Type L, which is the unusual type and Type H, which is what we were expecting our best identified by CT scan and are affected by different pathophysiological mechanisms. If not available, signs which are implicit in Type L and Type H definition could be used as surrogates: respiratory system elastance and recruitability. This elastance is basically the opposite of compliance. So if you have a very compliant lung you’re going to have low elastance. If you have a very high elastance, that would be a very low lung compliance. So to show you a little bit more about what he’s talking about, I wanted to go to another site and this site is developed by Dr.Paul Marik who about 3 or 4 years ago published a paper that heightened awareness of the role of vitamin C in septic shock. And there is a randomized controlled trial that is underway as we speak and we are waiting the results of that. But Dr. Marik is the Chief of pulmonary Critical Care Medicine at Eastern Virginia Medical School and he maintains a site that is constantly being updated in terms of his suggested approach to prophylaxis. Of course A lot of these things we don’t have randomized placebo-controlled trials for because we are in the midst of a pandemic. But I wanted to show you this because I think it’s a very good resource for those who are interested in learning more about how to ventilate patients and how to treat patients with COVID-19. Of course this is a pretty long document, but I want to show you what he has here on the site in terms of the L and the H phenotype. So the L phenotype as we said is the one that is unusual and you can see here that there’s not a lot of infiltrates on this CT scan of the chest. Here’s the patient laying on their back the top part here is their front and this is the sides of the patient here on the left, here on the right and we’re looking in their lungs which are black that should normally be black and anything that is white in this black background is abnormality. So you can see here, there’s not a lot of lung fluid and that’s why the compliance of this lung is near normal. Over here though, the type of phenotype that we were expecting to see the ARDS, you can see that there’s plenty of water and that these lungs are going to be very very stiff. So now for pathophysiology about what may be going on. Let’s take a little deeper look into the circulation of the heart and lungs and also ventilation to get a bigger understanding about what’s going on with phenotype L and H. So here we have the lungs and let’s say we’ve got an alveolus in one area of the lung and an alveolus and the other areas of lung. This is where oxygen and carbon dioxide are exchanged and breathing occurs. And this red thing here going on this side, and this red thing going on this side is the pulmonary artery going out to the alveoli and the pulmonary vein coming back.
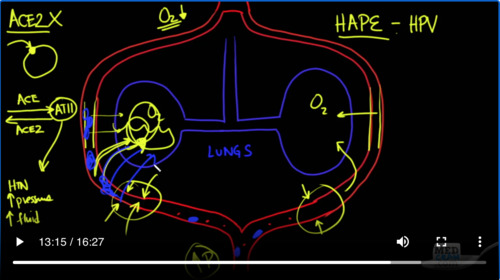
Basically, this is where little red blood cells are going to pick up oxygen and then carry it on. So if there were an ammonia, let’s say where there was pus or fluid in the alveolus. Then what would happen is oxygen coming in would not be able to get to this vascular structure the pulmonary capillary, And what would happen in that case is something called hypoxic pulmonary vasoconstriction or HPV and it has to do with the amount of oxygen here in the alveolus. It would signal to muscular cells pulmonary endothelium involved with smooth muscle and it would cause a constriction of this pulmonary artery and would cause less blood flow to go by here. Now that’s something that’s good to have happen because if there’s a certain portion of your lung that’s not getting oxygenation. You don’t want to have blood going to that area bypassing that area coming back and not picking up oxygen. It would be much better for that blood to be pumped to other areas of your lung which are getting ventilation and oxygenation. So hypoxic pulmonary vasoconstriction is a great mechanism to maintain oxygenation and essentially shut down areas of your lung which are not oxygenating well. Let’s say you’re going up to a higher altitude where oxygen is going to be at a premium. It’s going to be reduced. So you’re at say 12,000, 15,000 feet where oxygen is very low. Now that oxygen is low everywhere in every alveolus. It’s going to be low everywhere in the lungs what you get there a little can change is you get a global vasoconstriction of the pulmonary artery. And when that happens you get an increase in pulmonary artery pressure because the vasoconstriction is so global that it causes a back pressure and the pulmonary artery pressure in the heart goes up. Now that’s not a problem per se unless here at these very fragile capillaries and it’s very thin here. There’s a very thin membrane, it looks thick hair but there’s not, it’s a very thin membrane going across and these things can fracture, they can break because of the high pressure that’s in here and what you can get is actually leakage of fluid into the alveolar structures. And when that happens we call that high altitude pulmonary edema or HEIP. Now in this case high altitude pulmonary edema is because of hypoxic pulmonary vasoconstriction the same mechanism that we saw before is causing this vasoconstriction and it’s causing an obstruction to flow and increased pressures and causing fluid to come into the alveolus and not being able to oxygenate well. So key point here is that the reason why this is happening is because of lack of oxygen generally speaking, when you bring HEIP patients from high altitude down to a regular altitude because there’s more oxygen, these pathophysiological mechanisms resolve on their own and it’s very unlikely that somebody with HEIP would have to be taken to the Intensive Care Unit still have symptoms and need to be put on a ventilator like we’re seeing in COVID-19. In other words, these symptoms are pretty self–limiting. Once you get the oxygen up and you get the barometric pressure up, but the question remains is it possible that other things, other than lack of oxygen like we see in HEIP could be causing pulmonary vasoconstriction of these arteries causing an elevation in the pressure and leakage of fluid into the alveoli, and the answer is almost certainly “Yes, it’s possible”. In MedCram(update 37). We talked about the ACE2 receptor. But let’s just review a little bit, what we said and what we know is that when the SARS-CoV 2 virus comes into the cell. It’s actually binding to the ACE2 enzyme which is acting like a receptor that enzyme becomes down regulated. It is actually binding in the catalytic site for ACE2 and so ACE 2 essentially ceases to be able to exist. The more viruses you have, the more ACE2 gets knocked out of circulation according to the hypothesis. Ace which is a completely different enzyme goes toward something called Angiotensin II is opposed by ACE2 which actually causes vasodilation.
Angiotensin II does a lot of things. No 1, it causes hypertension, it can cause an increase in pressures in the lungs specifically. I can also cause an increase in fluid in the alveoli. So all of those things could be in play as well. Another thing that could happen is blood clots. If there are tiny blood clots. Micro thrombi which is what we’re beginning to suspect is happening. Those clots could block up the vasculature and that could also cause fluid to go in. In terms of the fact that it could be compared to HEIP or high altitude pulmonary edema. It’s certainly not that in the fact that when we give oxygen to these patients with high altitude pulmonary edema, they get better pretty quick and that’s certainly not the case with these COVID-19 patients. But the way that oxygen or lack of oxygen causes this HEIP like problem is with hypoxic pulmonary vasoconstriction. So something else that can cause vasoconstriction in the pulmonary artery is Angiotensin II which is broken down by ACE2, or it could be simulated with micro thrombi getting caught in there. So let’s take a look at the evidence for that. Here’s an article that was published in MEDPAGE TODAY: Anticoagulation Guidance Emerging for severe COVID-19 ——pragmatic choices dominate as guidelines are shaping up. They are noticing systemic clotting problems in patients with COVID-19. Even on those patients who have been on prophylactic anticoagulation at low levels. They’re noticing that a D-dimer which is a blood test that checks for clotting issues has been linked to substantially higher odds of death in hospitals among COVID-19 patients in Wuhan,China. Some are recommending that long chain or what we call unfractionated heparin, that would be through a Heparin drip would theoretically be preferable among anticoagulants because of their anti-inflammatory effects. The other option is using something called Low Molecular Weight Heparin. This is something that you can get injected into your abdomen for coverage, The DOACs have the least anti-inflammatory effects. These are the newer generation medications that you can take once a day or twice a day and you don’t have to have blood tests. So the one that would have the most anti-inflammatory effect would be the unfractionated heparin. Of course they have no data for any of this. But that’s where we are right now with COVID-19. We don’t have any hard data to say this is what we need to do. So why is this happening? Some of the theories is that there are antibodies that are being formed in the blood against these viruses foreign substances and that is causing this hypercoagulable state. They say here the presence of these antibodies may rarely lead to thrombotic events that are difficult to differentiate from other causes of multifocal thrombosis in critical patients such as disseminated intravascular coagulation, heparin induced thrombocytopenia and thrombotic microangiopathy. Something similar to what we were just talking about is the theory that the SARS-CoV-2 which binds to the ACE2 receptors may actually be causing inflammation in the micro vasculature and that’s causing platelet aggregation and thrombosis. They have no noticed that at autopsies there are inflammatory changes in the heart with fine interstitial mononuclear inflammatory infiltrates, but no viral inclusions meaning that there’s no viruses there. But yet there is still inflammatory infiltrates. Could these be related to clots unclear still at this point? So once again frustratingly more research needs to be done, but it’s a pathophysiological process that needs to be considered.
A:有些人可能需要使用呼吸机几个小时,而其他人可能需要一、两周或三周。如果一个人需要使用呼吸机更长的时间,可能需要气管切除术。在手术过程中,外科医生在颈部前部打个洞,并将一根管子插入气管。管子连接到呼吸机。气管切除术管插入声带下方,使说话困难。当患者从呼吸机中分离出时,他们可以使用一种叫做说话阀的设备重新开始说话。许多人可能可以使用呼吸机几个星期,试图从急性疾病中好转,但他们可能不愿意永久地使用呼吸机。许多人认为这是不能接受的。这就是为什么提前进行护理规划讨论对患者及其家人是好事。感染是与使用呼吸机相关的一种潜在风险;气道中的呼吸管可以让细菌进入肺部,从而导致肺炎。呼吸机也可以损害肺部,无论是由于压力过大或氧气水平过高,对肺部有毒。尤其是老年患者,其身体和认知功能最有可能下降。ICU幸存者可能觉得他们的思维和处理速度没有ICU之前那么快。例如,当你把某人带出他们的家庭环境,把他们放在一个陌生的地方,给他们一些他们通常不服用的药物时,会让他们面临更高的精神错乱风险。如果他们在 ICU 中出现精神错乱或需要镇静剂,这可能导致 ICU 停留后出现认知问题。患者也可能遇到心理健康问题,如PTSD[创伤后应激障碍]。尽管如此,呼吸机可以挽救生命,事实上,许多幸存下来的COVID-19严重病例的人不可能没有一个。



Add comment