新冠病毒药物: 瑞德西韦,氯喹,治疗试验(新冠肺炎讲座11)
Antiviral Medications, Treatment Trials (Remdesivir, Chloroquine) Lecture 11
Welcome to another MedCram coronavirus update. The deaths have gone from 427 to 493. That’s an increase of about 15%. That’s a little bit less than it’s been in the last couple of days. Normally it’s been running in the 18% to 20%. Confirmed infections have gone to 24,516; that’s up around 19%. And then we’ve got recovered; yesterday was at 657; today up to 906, and I think we should see that these numbers are going to continue to increase because we’ve gotten through a number of weeks of illness with these patients.
欢迎来到另一个MedCram冠状病毒更新。死亡人数从427上升至493。这增长了大约15%。这比过去几天少了一点。通常,它的运行速度为18%到20%。确诊感染病例为24,516;上升了约19%。然后我们康复了。昨天是657;今天到了906年,我认为我们应该看到这些数字将继续增加,因为这些患者已经经历了数周的疾病折磨。
Okay, we’re going to talk about some of the quick items in the news, and then we’ll get to the big topic, which has been new drugs that are being looked at to treat the coronavirus pneumonia and lung diseases.
好的,我们将快速谈论新闻中的一些话题,然后我们将讨论一个大话题,即正在研究用于治疗冠状病毒性肺炎和肺部疾病的新药。
Okay, quick items in the news. First of all, testing in the United States has typically been done by the CDC up to this point. Well, now they are going to distribute those two specific local labs so that those labs can process some of these tests that could be done perhaps on a more regular basis. This is the swab up to this point. There have been eleven positive out of 178 tests that have been performed. There are another 82 tests that are outstanding right now, and we’ll see what the results of those show.
好的,新闻中的快速内容。首先,到目前为止,CDC通常在美国进行测试。好吧,现在他们将分发这两个特定的本地实验室,以便这些实验室可以处理其中一些测试,这些测试可能会更定期地进行。这是到目前为止的拭子。 178项测试中有11项阳性。目前还有另外82项出色的测试,我们将看看这些结果显示了什么。
Yesterday, we talked about the Diamond Princess; about 3700 are on board. And this is floating off the coast of Japan, and it looks as though there are 10 positive onboard based on the stories that are coming out. Remember this was a ship that had sailed, and it was found out that there was a man that was on this ship in late January.
昨天,我们谈到了钻石公主。船上约有3700架。而且这是漂浮在日本沿海的,根据即将出现的故事,似乎有10项积极的建议。请记住,这是一艘航行的船,发现一月下旬有一个人在这艘船上。
And 10 of them are positive already on the ship. Unclear if it was a direct result of this man that had tested positive earlier. Thirdly. There is another dashboard out there. I know we’ve had some positive feedback for the Johns Hopkins dashboard. I wanted to make you aware of another dashboard that I thought was very clean, had a lot of good information on it. And so we’ll put a link in the description.
其中有10艘已经在船上呈阳性。不清楚这是否是该人早先测试阳性的直接结果。第三。那里还有另一个仪表板。我知道我们已经对Johns Hopkins仪表板获得了一些积极的反馈。我想让您知道另一个我认为非常干净的仪表板,该仪表板上有很多很好的信息。因此,我们将在说明中添加一个链接。
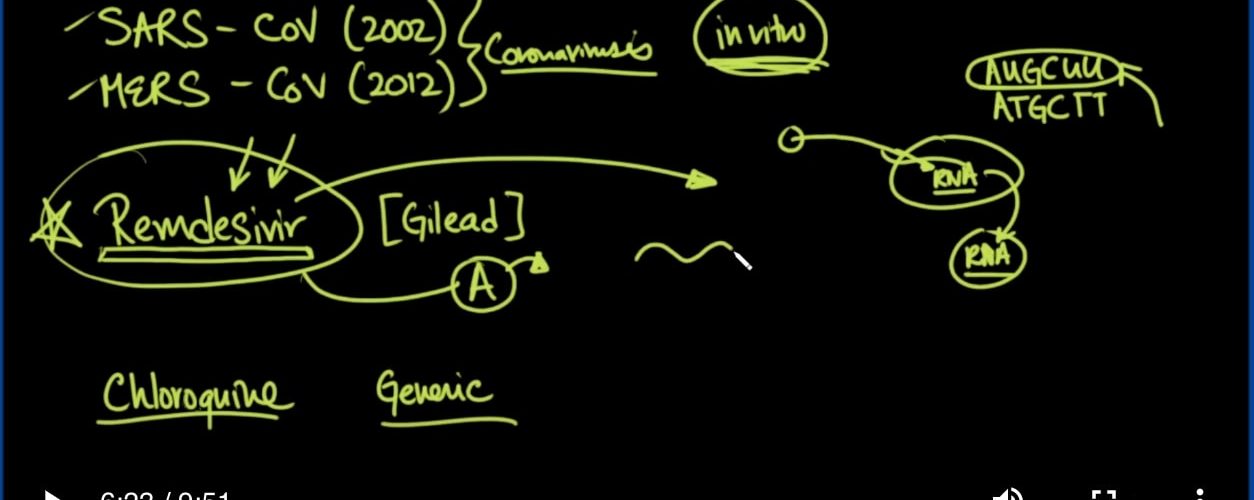
Okay. So there’s this letter to the editor to Nature. Nature’s of big journal, very prestigious. This is February the 4th that this was published, and it is describing the medications specifically Remdesivir and Chloroquine.
好的。因此,这封信是写给《自然》编辑的。大自然杂志,非常有声望。这是2月4日发表的文章,它描述了药物瑞德西韦和氯喹。
And why this is important? So let’s talk about Remdesivir first. Going back, there were a number of medications that were looked at during the SARS and MERS outbreaks. Both of which by the way are coronaviruses. There were medications that they had tried like Rebrivin. Obviously, they had tried Remdesivir as well.
为什么这很重要?因此,让我们先谈谈瑞德西韦。回过头来,SARS和MERS爆发期间曾看过许多药物。顺便说一下,两者都是冠状病毒。他们尝试过像Rebrivin这样的药物。显然,他们也尝试过瑞德西韦。
And these were shown to be efficacious in stopping viral production in vitro. So the key there is the word in vitro. In vitro means in a Petri dish, in a test tube, not necessarily in a human being. And this is where a lot of tests are done at first.
并且这些被证明在停止体外病毒产生方面是有效的。因此,关键在于体外。体外是指在皮氏培养皿中,在试管中,而不是人类。这是首先要进行大量测试的地方。
Now, what they did do is they looked at this medication called Remdesivir, and they tried it in Ebola. The reason why they did this was because it worked in Ebola. It worked in vitro in Ebola, and they said hey, maybe we can try this in Ebola patients, or in people who are in proximity to people with the Ebola virus so that they don’t get Ebola.
现在,他们所做的就是看了这种名为瑞德西韦的药物,并在埃博拉病毒中尝试过。他们之所以这样做,是因为它在埃博拉有效。它在埃博拉病毒中体外起作用,他们说,嘿,也许我们可以在埃博拉病毒患者或与埃博拉病毒患者接近的人们中尝试这种方法,以使他们不会感染埃博拉病毒。
So, when they tried it, it didn’t work. Okay, people still got Ebola. So that’s an example where a medication works in vitro, but not in vivo. Nevertheless, before they could take this medication Remdesivir to market, before they could even try it in these patients. They have to make sure that it was safe. And so the safety tests have already been done on Remdisivir. We know that it’s a relatively safe medication to use in patients. The problem was it just didn’t work in vivo in patients in real life, but it did work in vitro against a bunch of viruses.
因此,当他们尝试时,它不起作用。好的,人们仍然感染了埃博拉病毒。因此,这是药物在体外有效但在体内无效的一个例子。尽管如此,在他们将雷姆昔韦推向市场之前,他们甚至还没有对这些患者进行尝试。他们必须确保它是安全的。因此,安全性测试已经在瑞德西韦上完成。我们知道这是一种用于患者的相对安全的药物。问题在于,它在现实生活中对患者没有体内作用,但对多种病毒却有体外作用。
So enter in now, the coronavirus 2019, and you’ve got a situation where people are dying, and there’s an epidemic. Why not try Remdesivir? Well, the first thing you got to want to do is to make sure this medication actually works against not the SARS, not the MERS, not Ebola, but the actual coronavirus 2019. And so that’s what they did. They actually tried it in cells that were infected with the current strain of the coronavirus from Wuhan, China.
因此,现在进入2019年的冠状病毒,您会遇到人们正在死亡的情况,并且有一种流行病。为什么不尝试瑞德西韦?好吧,您要做的第一件事就是确保这种药物实际上对付SARS,MERS,埃博拉而不是SARS,而是真正的2019年冠状病毒。这就是他们所做的。他们实际上在感染了目前来自中国武汉的冠状病毒株的细胞中进行了试验。
Now, how does this medication work? What does it do? So you’ve got the cell, and you’ve got the virus, and the virus has to fuse with the cell, and it goes inside the cell, and when it does that, it basically dumps its RNA into the cell and uses the cellular machinery to reproduce more RNA.
现在,这种药物如何起作用?它有什么作用?因此,您有了细胞,又有了病毒,并且病毒必须与细胞融合,然后病毒进入细胞内部,当病毒进入细胞时,它基本上将其RNA倾倒入细胞并使用细胞机器可复制更多RNA。
Well, for those of you who are aware of what RNA is or DNA for that matter, it’s basically just the code. You know, the DNA code, for instance, is like ATGC, and this can go on and on and on. With, however, RNA, instead of having the Ts, those Ts become Us. So instead of Ts, you’d have AUGCUU. Okay, that’s the only real difference, and of course, it’s usually single-stranded, and DNA is usually double-stranded.
好吧,对于那些知道什么是RNA或DNA的人,基本上只是代码。您知道,例如,DNA代码就像ATGC,并且可以不断地进行下去。但是,有了RNA,这些T就会代替我们而变成Ts。因此,您将获得AUGCUU,而不是Ts。好的,那是唯一的真正区别,当然,它通常是单链的,而DNA通常是双链的。
So what happens is that these nucleotides are lined up one after the other, and there are, you know, tRNAs that come in and help put the nucleotides in the correct order, and this is done using different polymerases.
因此,发生的事情是这些核苷酸彼此排成一行,并且,您知道,有tRNA进入并有助于按正确的顺序排列核苷酸,这是使用不同的聚合酶完成的。
Well, what this medication does is it resembles one of the As, except it doesn’t allow the next one to come on, whatever that happens to be. So the virus comes into the cell, and this is a Ha, I’m taking you over, and you’re going to start to produce more RNA viruses like me, and so the machinery starts to go along, and as soon as it gets to an A, this medication gets put in and boom. It’s done. There’s no more polymerization. The strand cannot continue, and so this arrests the production of virus.
嗯,这种药物的作用类似于As之一,不同之处在于它不允许下一种药物出现。因此,病毒进入了细胞,这就是哈,我要带你过去,您将开始像我一样产生更多的RNA病毒,这样机器就开始运转了,到达A时,这种药物就会放进去并蓬勃发展。完成。不再聚合。该链无法继续,因此这会阻止病毒的产生。
Well, the good news is that they found that this worked well in the new 2019 strain of the coronavirus, and so, here you’ve got a medication that is already been tested on human beings for safety. It’s available. It’s there. You just don’t have a good FDA indication because it doesn’t work on Ebola, and you don’t have SARS outbreak currently, but now you’ve got this coronavirus outbreak.
好吧,好消息是他们发现这种方法在2019年新的冠状病毒株中效果很好,因此,这里有一种已经过人类安全性测试的药物。有空在那。您只是没有良好的FDA适应症,因为它不适用于埃博拉病毒,并且您目前没有SARS爆发,但是现在您已经出现了这种冠状病毒爆发。
So what do they do? Even though they don’t have any evidence that it’s going to work, and usually we only give medications to people when we have evidence that it works, they use it for something called compassionate use, in other words, look, this patient’s going to die, let’s give them this medication see if it works. So they did that, they did that in the first patient in the United States that came down with the virus, and it was in Washington state, and within a day this patient started feeling better, and within three or four days the fever completely run away.
那他们怎么办?即使他们没有任何证据表明它会起作用,并且通常我们只会在有证据证明它起作用时才向人们提供药物,但他们仍将其用于有同情心的用途,换句话说,看一下,该患者会死了,让我们给他们这种药看看是否有效。因此,他们这样做了,是在美国首例感染这种病毒的患者中进行的,当时是在华盛顿州,一天之内,患者开始感觉好些,三,四天内发烧完全消失了。远。
And we’ve got a number of articles that talk about this, and we’ll put those in the links. So this could be a big breakthrough here if this works, but remember, want to make sure you understand that this medication has never worked in vivo.
我们有很多关于此的文章,并将它们放在链接中。因此,如果可行,这可能是一个重大突破,但请记住,要确保您了解这种药物在体内从未起作用。
So we don’t know if it’s going to work. So what they’re planning on doing here coming up very shortly is actually they are going to undergo a trial in China where they give some people Placebo, and they give others this Remdesvirir there and see whether or not it works. We should know by the end of April whether or not this thing is going to work.
因此,我们不知道它是否会起作用。因此,他们计划很快在这里进行的操作实际上是要在中国进行试验,他们给一些人安慰剂,然后在这里给其他人瑞德西韦,看看它是否有效。我们应该在4月底之前知道这件事是否会奏效。
Now, as you can imagine, this is cause some store. The company is Gilead that makes this, and I’m sure if you look at their stock prices, they are probably going up in the hopes that this thing works, and that the epidemic can be abated.
现在,您可以想象,这会引起一些麻烦。该公司就是吉利德(Gilead)的公司,我敢肯定,如果您看看他们的股价,他们可能会上涨,希望这件事行得通,并且可以消除流行病。
So the other drug that they looked at in the same letter to the editor, and that study was chloroquine. Now chloroquine has been around for a very long time. It’s a generic medication. It’s been used in malaria for years. In fact so much so that you actually have chloroquine resistance areas.
因此,他们在给编辑的同一封信中看到的另一种药物是氯喹。现在氯喹已经存在很长时间了。这是一种通用药物。它已经在疟疾中使用了多年。实际上如此之多,以至于您实际上具有氯喹抗性区域。
They also found that this work really well as well. So how does that work? Well, you’ve got the cell, you’ve got the virus. There’s a special PH that these lysosomes have to be before these things can merge, and what chloroquine does is it raises the PH of those lysosomes, preventing these things from merging, so it’s possible that you could prevent against viral particles from infecting other cells.
他们还发现这项工作也很好。那如何运作?好吧,你有细胞,你有病毒。这些溶酶体必须具有特殊的PH才能融合在一起,而氯喹的作用是提高这些溶酶体的PH值,从而防止这些东西融合,因此有可能可以防止病毒颗粒感染其他细胞。
Now these are just two drugs that are being looked at. There are the Pinonabir and Ratonabir. These are other medications that are being used. In fact, they were used in Thailand, and they were thought to improve.
现在,这些只是正在研究的两种药物。有皮诺纳比和拉托纳比。这些是其他正在使用的药物。实际上,它们在泰国使用,并且据认为可以改进。
The problem is with any of these medications that are used for compassionate use, if you use them, and the patient gets better, you don’t know if that was the reason why they got better, or if it was some other reason they got better. That’s why you need to do a randomized, placebo-controlled trial。
问题在于这些用于同情的药物,如果您使用它们,并且患者病情好转,您不知道这是为什么病情好转的原因,还是其他原因更好。因此,您需要进行随机,安慰剂对照的试验。
Okay, so this is the latest hot-off-the-press information regarding coronavirus. If you found this is helpful, you found this channel is helpful, please subscribe and that will help us make more of these videos and continue the updates as this saga unfolds. Thanks for joining!
好的,这是有关冠状病毒的最新热销信息。如果您发现此频道对您有帮助,则认为该频道对您有所帮助,请订阅该频道,这将有助于我们制作更多这些视频,并随着故事的发展而继续进行更新。感谢您的加入!



Add comment